Abstract
Monosomy 7 and deletion of chromosome arm 7q (-7/-7q) occur in up to 10% of patients with acute myeloid leukemia (AML). In AML, -7/-7q are associated with resistance to chemotherapy and adverse prognosis. As such, treatment outcomes for this subgroup of patients remain particularly dismal, and targeted therapies are urgently needed.
To identify targeted therapies for AML with -7/-7q we analyzed ex vivo drug sensitivities in 158 AML samples. The presence of -7/-7q (n=18) was determined using karyotyping and exome-sequencing of bone marrow (BM) mononuclear cells and a matched skin biopsy control. Mononuclear cells from fresh bone marrow aspirates of AML patients were incubated with a library of up to 525 oncology drugs for 72h, with each drug pre-plated in 5 concentrations. Cell viability was measured using the CellTiter Glo (CTG) assay, and the response of each sample was summarized as a drug sensitivity score (DSS), which is a modified integration of the dose-response inhibition curve, directly proportional to the sensitivity of a sample.
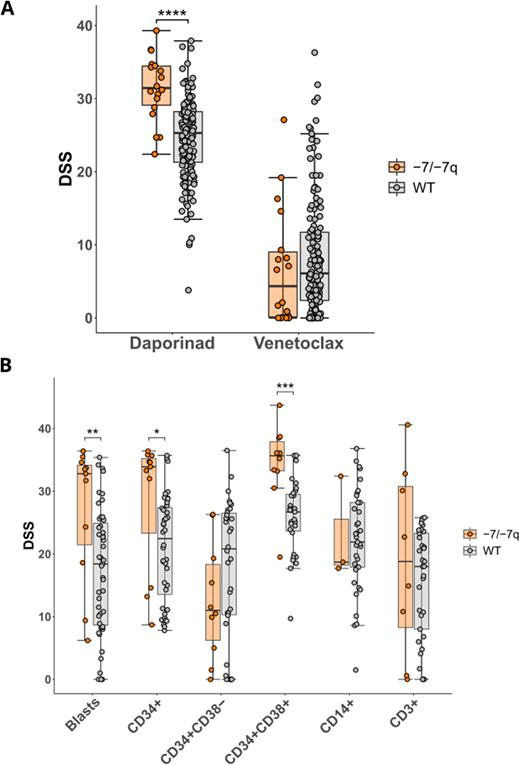
We found that AML samples with -7/-7q were highly sensitive to the nicotinamide phosphoribosyltransferase (NAMPT) inhibitor daporinad (p-value ~ 4.2e-06) (Figure A). NAMPT is the rate-limiting enzyme in the NAD+ salvage pathway. The NAMPT gene is located at 7q22.3. Monosomy 7 and del(7q) lead to the deletion of one copy (i.e., haploinsufficiency) of NAMPT. Notably, a rare AML with del(7q) in which the deletion did not include the NAMPT gene (and which was thus diploid for NAMPT) was not sensitized to NAMPT inhibition. Taken together, this data demonstrates a significant association between the NAMPT haploinsufficiency in AML with -7/-7q and increased sensitivity to NAMPT inhibition.
To further support our findings, we analyzed the sensitivity of AML cells without or with -7/-7q to four NAMPT inhibitors (daporinad, GMX1778, KPT-9274, and LSN3154567) using multiparametric flow cytometry. Viably frozen BM mononuclear cells from AML patients with (n=11) and without (n=51) -7/-7q were thawed and incubated with drugs in 8 concentrations for 72 hours. After incubation, cell viability was analyzed using the iQue Screener Plus flow cytometer with a panel consisting of CD45, CD3, CD34, CD117, CD14, and CD38 antibodies, to identify myeloid and lymphoid cell populations, as well as Annexin V and 7-AAD to discriminate live from dead cells. We found that CD34+ myeloblasts from AML with -7/-7q were selectively killed by all four NAMPT inhibitors, validating our initial results (p-value ~ 1.4e-02). An in-depth analysis of cellular subpopulations revealed that while CD34+CD38+ cells (representing more differentiated myeloblasts) were significantly more sensitive to NAMPT inhibition in -7/-7q samples compared to wild-type samples (p-value ~ 4.5e-04), the CD34+CD38- compartment (anticipated to contain the immature leukemic stem and progenitor cells) was not sensitized in this group (Figure B).
Interestingly, the analysis of ex vivo drug sensitivities also revealed that while the majority of AML with -7/-7q are resistant to the FDA-approved BCL2 inhibitor venetoclax, they are highly sensitive to daporinad (Figure A). This finding proposes NAMPT inhibitors as a suitable alternative to venetoclax for -7/-7q patients. Additionally, since venetoclax has previously shown significant efficacy against the highly BCL2-dependent undifferentiated (CD34+CD38-) myeloblasts, its combination with NAMPT inhibitors represents a promising strategy for achieving total leukemic cell death in these patients.
In conclusion, we have found that AML with NAMPT haploinsufficiency caused by -7/-7q shows selective sensitivity to NAMPT inhibition. This suggests that NAMPT inhibitors have the potential to be a potent targeted therapy for AML with -7/-7q, a disease with a particularly adverse prognosis.
Disclosures
Gjertsen:BerGenBio: Consultancy; Pfizer Inc: Consultancy; Alden Cancer Therapy: Current holder of stock options in a privately-held company; KinN Therapeutics: Current holder of stock options in a privately-held company; Novartis: Consultancy. Kontro:Novartis: Membership on an entity's Board of Directors or advisory committees; Faron Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas Pharma: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees. Porkka:Pfizer: Honoraria; Celgene/Bristol-Myers Squibb: Research Funding; Incyte: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Novartis: Honoraria; Incyte: Honoraria; Bristol-Myers Squibb: Honoraria; Astellas: Honoraria; AbbVie: Honoraria. Heckman:Oncopeptides: Research Funding; IMI2 projects HARMONY and HARMONY PLUS: Research Funding; WntResearch: Research Funding; Orion: Research Funding; Kronos Bio: Research Funding; Novartis: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal